pharmaceutical production, tablet integrity, shape, and color consistency are essential for product quality and regulatory compliance. BT Print’s AI-powered Tablet Inspection System ensures every blister pack or ALU-ALU strip is visually inspected in real-time, detecting missing, broken, or miscolored tablets before the product reaches the consumer.In
This cutting-edge system is designed to integrate seamlessly with high-speed blister packaging and ALU-ALU machines, ensuring zero-defect output and reducing reliance on manual checks.
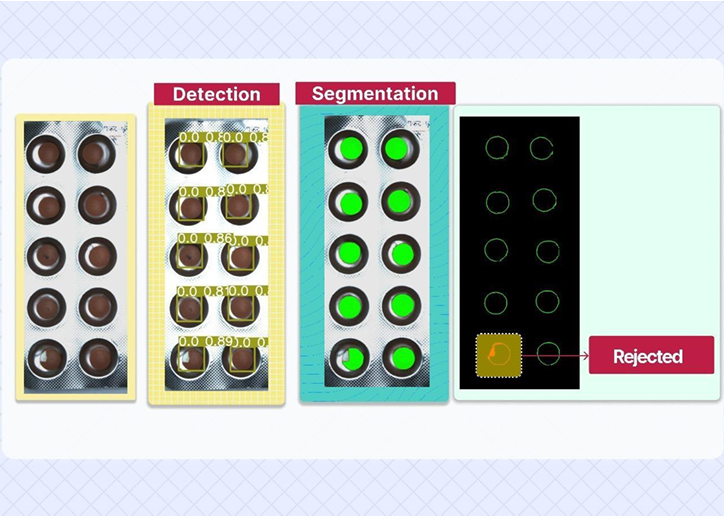
How It Works:
- High-resolution industrial cameras capture each tablet strip in real time.
- AI segmentation models isolate each tablet inside the packet—regardless of layout or orientation.
- The system analyzes:
- Tablet presence
- Shape conformity (detects broken, chipped, or malformed tablets)
- Color consistency (flags discoloration or incorrect coatings)
- Any deviation from the pre-trained standard results in instant rejection of the strip or packet.
Key Applications:
- Pharmaceutical Blister Packaging Machines
- ALU-ALU Strip Inspection Systems
- High-speed oral solid dosage production lines
Key Features & Benefits:
- AI-Based Tablet Segmentation – Accurately identifies and segments each tablet in complex layouts
- Shape & Color Verification – Detects defects based on predefined shape profiles and color tolerances
- High-Speed Inline Inspection – Designed for modern pharma lines with minimal disruption
- Real-Time Rejection – Immediate removal of faulty strips using rejection modules
- Trainable AI Models – Customizable for various tablet types, shapes (round, oval, capsule), and coating styles
- Batch-Wise Analytics – Track defect rates, rejection patterns, and compliance reports
- 21 CFR Part 11-Ready Logs – Suitable for regulated environments requiring audit trails
- Reduces Manual Inspection Load – Increases efficiency and reduces human error
This system helps pharmaceutical manufacturers achieve consistent product quality, minimize recall risks, and maintain compliance with regulatory standards